Photocatalytic Water Reduction, Syntheses, Kinetics and Mechanisms
Lead by Prof. Roger Alberto
Solar light-driven water or proton reduction relies on catalysts. Since the process of H2 formation includes two electrons and two protons, their metal cores and/or associated ligands must accommodate reversibly at least two electrons. We focus our research on stable and long-lived catalysts mainly on cobalt and acyclic or cyclic poly-pyridyl ligands. Besides longevity, physico-chemical characteristics such as overpotentials and turnover frequencies are crucial factors and demand tailor-made chelators. Subtle alterations in the ligand sphere may have dramatic effects on performances.
We started with tetra-pyridyl ligands and the corresponding cobalt complexes (Inorg. Chem. 2011, 10.1021/ic102317u), proceeded to pentadentate systems of various structures (Chem. Commun. 2016, 10.1039/c4cc01500b) and closed the pyridyl framework to get macrocyclic, porphyrin-like systems fully based on pyridine (Inorg. Chem. 2018, 10.1021/acs.inorgchem.7b02992).
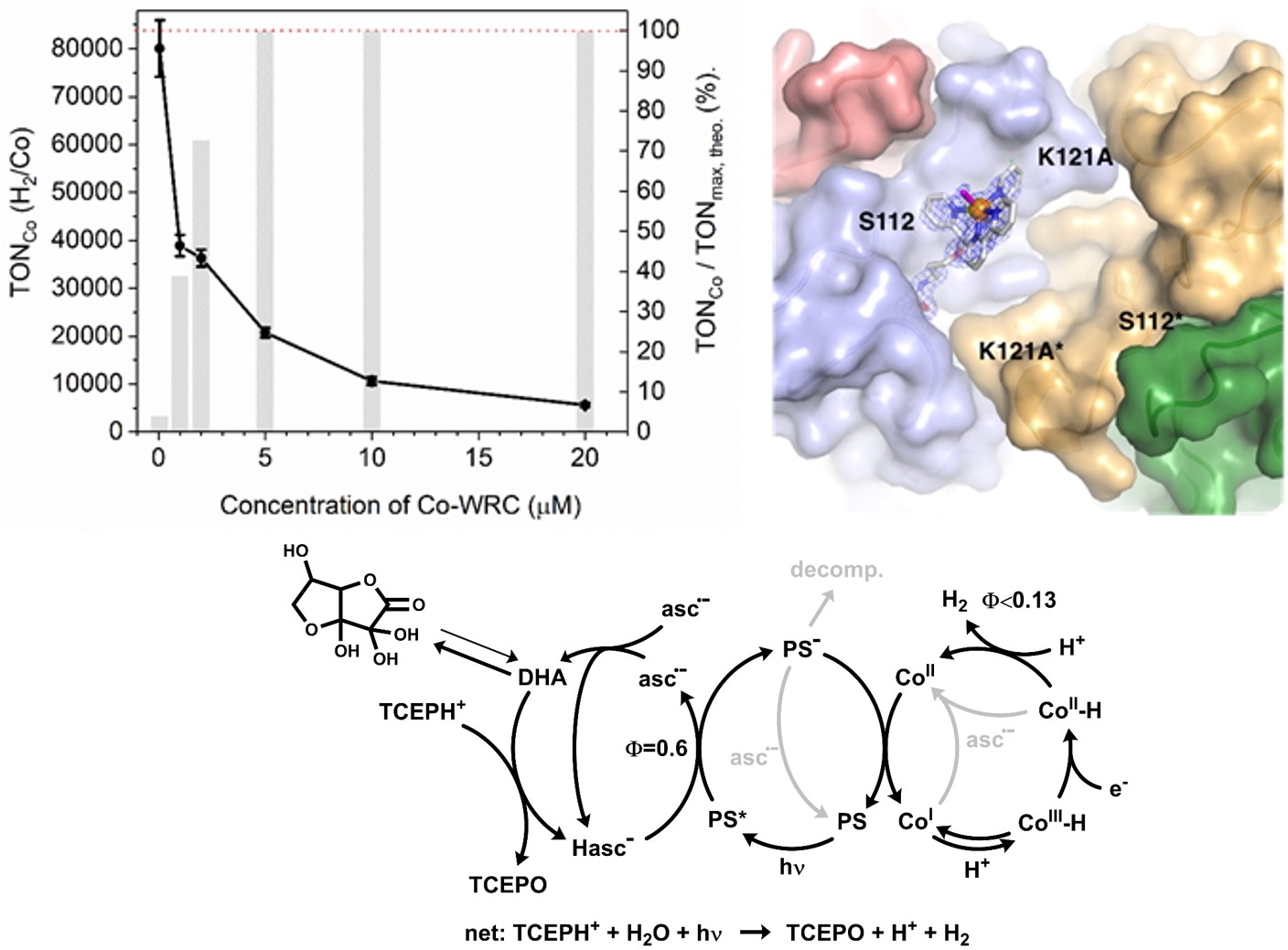
Each of the complexes had its advantages and disadvantages but turnover numbers increase from a few tenths to more the one hundred thousand. For each of the catalysts, a thorough mechanistic elucidation is mandatory for learning which factor has to be fine-tuned (Inorg. Chem. 2015, 10.1021/ic502591a). Electrochemistry, XPS and X-ray structure analyses deliver further important information. We investigate which factors limit performance (ChemSusChem, 2017, 10.1002/cssc.201701511). For our best catalysts, the photocycle, i.e. the photosensitizer, and no longer the catalyst, is performance limiting. Thus homogeneous experiments allow for a detailed analysis but do not tell us about the stability of our best water reducing catalysts. Thus, we proceed with promising catalysts to surfaces, which demands incorporation of surface-affine groups (Catal. Sci. Technol. 2020, 10.1039/d0cy00330a). Also, one catalysts was incorporated in a biotin/streptavidin system. This artificial metalloenzymes exhibited performance comparable to the free catalyst (Helv. Chim. Acta, 2018, doi:10.1002/hlca.201800036).
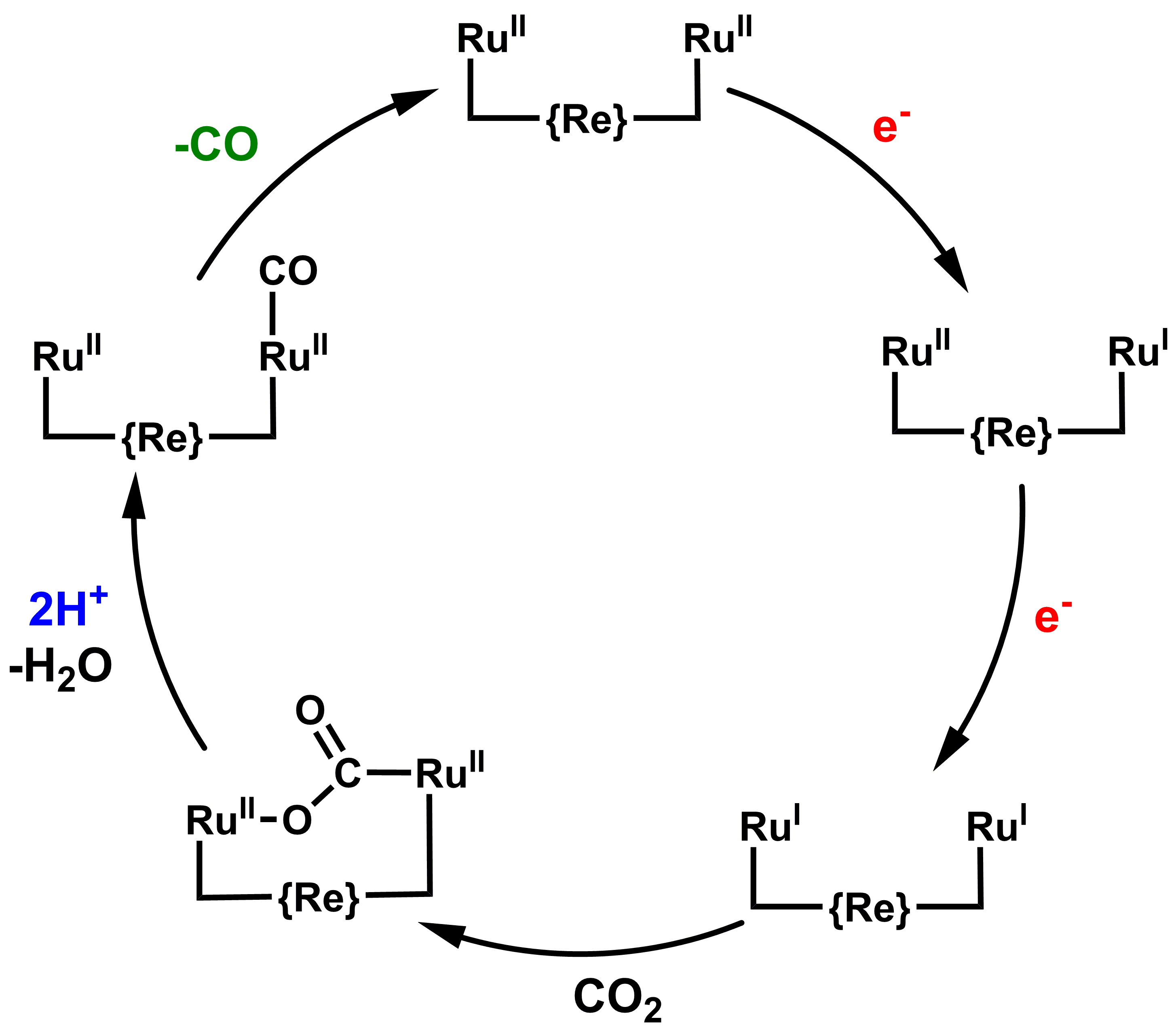
The reductive process of converting protons to di-hydrogen can be adapted to CO2 to CO reduction, although rarely done with cobalt. Indeed, some of our catalysts are equally good for this process and specially designed systems do this with high selectivity. Besides cobalt, ruthenium is a well-known catalyst for CO2 reduction and we recently showed a dinuclear complex to display a concerted electrocatalytic mechanism for this two-electron two-proton process (Helv. Chim. Act. 2020, 10.1002/hlca.202000147).
The development of new catalysts for water reduction and their combination with a water oxidation process is the ultimate goal of the research in this part of the LightChEC consortium. This research is done in collaboration with groups from pyhsics, physical chemistry, the Empa and other collaborations.