Timeresolved Spectroscopy of Artificial Photosynthetic Systems
Lead by Prof. Peter Hamm
Any chemical reaction cycle may, in principle, be elucidated by time-resolved spectroscopy, provided that its intermediates are transiently populated in sufficient amounts, and that they exhibit characteristic marker bands. Just like natural photosynthetic systems, the artificial photosynthetic systems, which are developed in this project, are perfectly suited to be investigated with essentially unlimited time-resolution by optical spectroscopy, as they are naturally photo-triggerable.
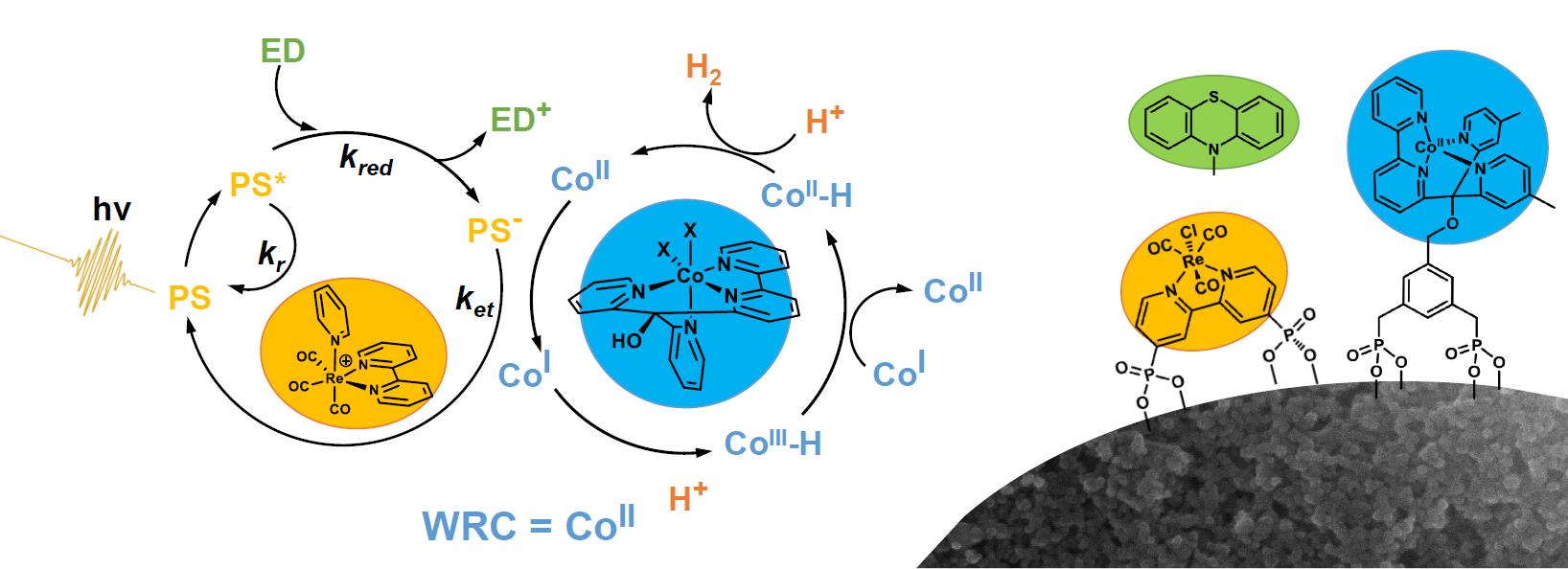
Water splitting is typically divided into its two half reactions, hydrogen evolution or water reduction vs oxygen evolution or water oxidation. In homogenous solution, water reduction systems with reasonable yields and turn-over-numbers have been developed in the group of Roger Alberto in the first half of LightChEC, and its complex reaction cycle has been fully elucidated by transient IR and UV/VIS spectroscopy (see Figure below, left side). However, it seems inevitable that coupling to the oxidative half reaction will require some sort of compartmentation, since too many very reactive redox-partners would coexist otherwise. We currently focus on functionalized surfaces as one possibility of compartmentation, immobilizing the same molecules as previously studied in homogeneous solution (see Figure below, right side).
In homogenous solution, the reaction cycle can be controlled kinetically. That is, reaction rates are diffusion controlled, and can be tuned by concentration. Assembly of reaction partners on a surface enables much higher local concentrations of the reaction partners, and therefore may lead to significantly faster reaction rates. However, unwanted side reactions can speed up as well. We learned that the naive transfer of a water reduction system, which has been optimized in homogenous solution, onto a surface will likely not work, since the kinetics of the various reaction steps change by too much. A working water reduction system on a surface will require different concepts. One possibility is to invoke a semi-conducting substrate as mediator for charge transfer and charge diffusion. Alternatively, we will have to find methods to take more control over the surface arrangement of the reaction partners to better control the kinetics. Both approaches are a playground for a lot of very beautiful science