Electronic Structure of materials for solar-water splitting
Lead by PD A. Borgschulte
The field of artificial photosynthesis and especially solar water splitting has experienced ever-increasing interest from the scientific community over the past decades due to its potential to provide means to environmentally and economically sustainable energy storage framework. However, many mysteries remain. For example, obtaining operando spectroscopic information is still a great challenge due to various technical challenges. Our focus is in developing accessible spectroscopic techniques for a comprehensive understanding of the electronic structure and its dynamics in materials for solar water splitting. This includes the electronic structure of single molecules as well as extended solid frameworks. Our approach centres around using operando techniques, especially, photoelectron spectroscopy and magneto-optical spectroscopy.
The former is the membrane-based approach for X-ray photoelectron spectroscopy (XPS) analysis of systems at ambient pressure. This includes, but is not limited to, the reactions in aqueous environment. The membrane device, which is composed of a polymer impregnated with a photocatalytic system for water splicing, is required to bridge the desired analytical pressure of 1 bar to the ultra-high vacuum (UHV), which is required for the XPS analysis. Thus, the membrane is exposed to ambient pressure gas on one side and to UHV on another (Figure 1).
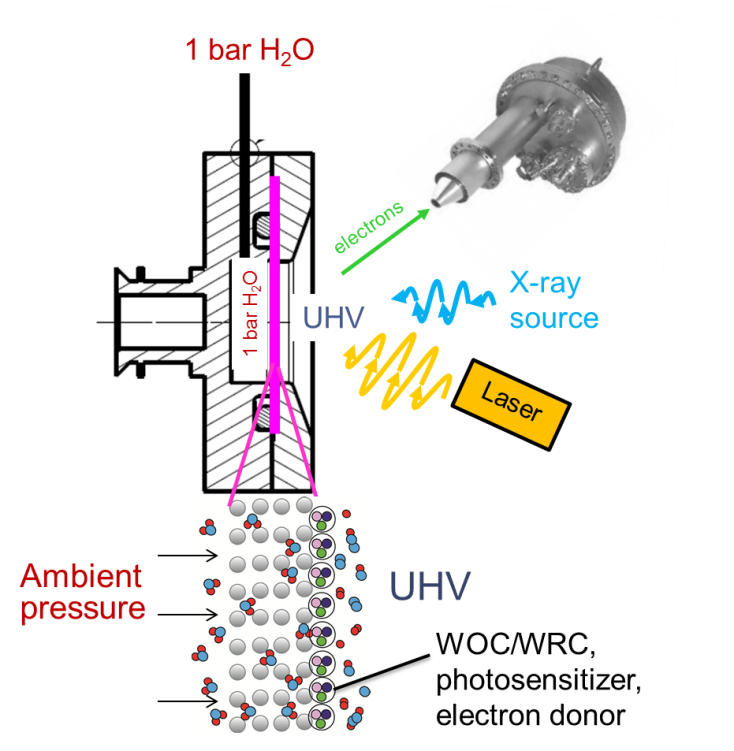
Controlling flux kinetics of the gas from ambient pressure to UHV side can yield desorption, rather than diffusion, limited flux. In this case, the concentration in the membrane is uniform throughout, i.e., same as at the ambient pressure side. Therefore, the analysed membrane surface (few nm) is at the desired pressure of 1 bar. The applicability of this approach has been demonstrated by our group for hydrogen chemisorption. Currently, the system is adapted to measure heterogeneous solar water splitting systems.
A different technique, time resolved magneto-optical spectroscopy (trMOKE), is developed in our lab for studying homogeneous as well as heterogeneous solar water splitting systems. Here, we take advantage of the magneto-optical phenomena of matter (see Figure 2).
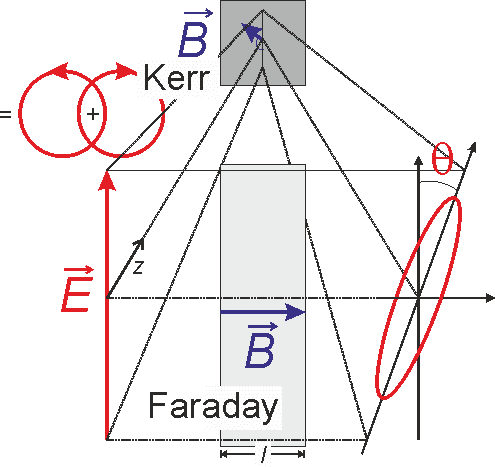
As magnetic properties are often indicative of and directly related to the electronic changes, trMOKE provides operando analysis of changes in the electronic structures of compounds of interest. We were able to demonstrate the proof of concept that changes of the oxidation number of d-metal ions of the water reduction catalysts dissolved in water can be followed using the Faraday configuration. In this project, the principle will be further developed including the investigation of solid electrode – electrolyte interfaces.
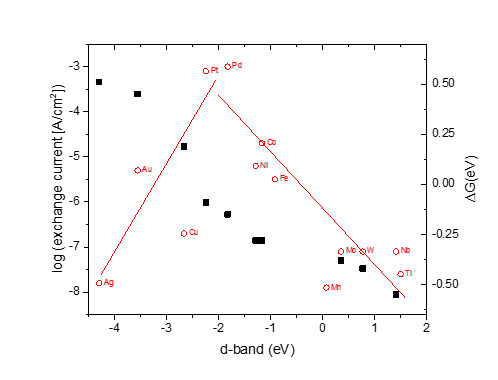
Apart from empirical investigations, important task is the modelling of the data. In catalysis, established models exist which are used to anticipate the activity of catalytic surfaces (e.g., the Norskov model). The work includes the definition of key parameters which are accessible by experiment such as the exchange current by electrochemical measurements and the (shift of the) d-band center by XPS (see Figure 3 for the in water electrolysis, from Norskov et al.). This will help in finding the rate-limiting step aiming at an optimization of the catalysts.