Catalysts for CO2 conversion into liquid sunlight
Lead by Prof. K.H. Ernst
It is paramount to reduce the dependence on fossil energy and to control CO2 emissions by developing renewable energies. One of the most attractive strategies is to close the carbon cycle by producing “liquid sunlight” by transforming CO2 into liquid fuel. Thus, much attention is paid to the hydrogenation of CO2 into safe and transportable liquid hydrocarbons, including alcohols and gasoline. Heterogeneous catalysis is the science and technology of transforming molecular structures by using a solid functional material (“the catalyst”) to control the energy profile and pathway of a reaction. This control enables the direction and selectivity of the reaction to be determined. Because all processes in large scale productions must be optimized away from today’s crude-oil based technology, development of new and more effective catalysts must follow.
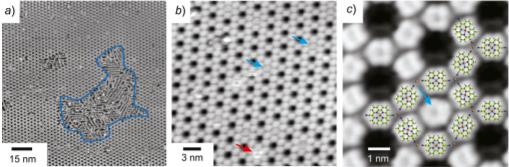
Figure: Scanning tunneling micrograph of a porous 2D Cu pyrphyrin MOF (Christian Wäckerlin, Empa)
Metal-organic frameworks (MOFs) and their two-dimensional (2D) or thin film equivalents are promising candidates as heterogeneous catalysts. MOFs are known for their specific interactions with gas molecules; this, combined with their rich and ordered porosity, makes them promising candidates for the photocatalytic conversion of gas molecules to useful products. In particular the fact that single metal atoms are stabilized in MOFs and that they can act as active sites in heterogeneous catalysts is promising. If relatively small ligands are used, MOFs will provide a high density of active sites.
In this project, we will focus on the on-surface synthesis of MOFs in ultrahigh vacuum on metal surfaces and on thin oxide surfaces. Their catalytic activity will be tested in-situ by advanced surface spectroscopies. As carbon dioxide is a linear molecule, the identification of a bent CO2 species, for example, is a good indicator for its activation. In a later phase of the project, the MOF samples will be used as electrodes in the liquid phase and tested for their electrocatalytic activity.
The oxygen evolution reaction has an important role in many alternative-energy schemes because it supplies the protons and electrons required for converting renewable electricity into chemical fuels. Electrocatalysts accelerate the reaction by facilitating the required electron transfer, as well as the formation and rupture of chemical bonds. This involvement in fundamentally different processes results in complex electrochemical kinetics that can be challenging to understand and control, and that typically depends exponentially on overpotential. At that point, 2D MOFs are hoped to become a new class of highly efficient electro catalytic materials.