Speakers and Abstracts
Table of contents
Carolina Gimbert Suriñach
| Departament de Química, Universitat Autònoma de Barcelona, ES | |
 |
Carolina Gimbert Suriñach obtained her PhD from the Autonomous University of Barcelona (UAB) in 2008 under the supervision of Prof. M. Moreno-Mañas and Prof. A. Vallribera, working on the development of new sustainable organocatalytic processes. After one year as an assistant professor at the same university, she moved to the University of New South Wales to undertake postdoctoral research in the field of bioinorganic chemistry. In 2012 she started a second postdoctoral position at the Institute of Chemical Research of Catalonia in the group of Prof. A. Llobet. During this time, she developed hydrogen evolution and water oxidation photocatalytic systems. In 2015 she was promoted to scientific group coordinator and her research focused on implementing molecular catalysis into water-splitting devices. After a short stay in the University of Barcelona, in early 2021 she joined the Chemistry Department of UAB as a Ramón y Cajal researcher and professor. |
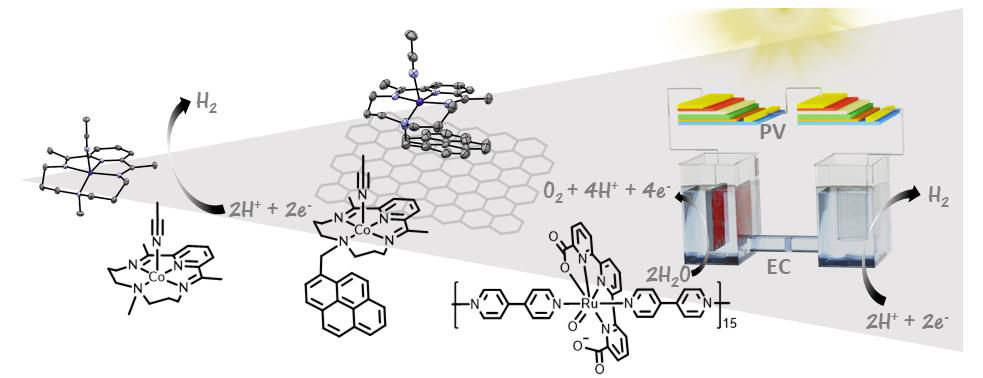
Molecular Systems for Water Splitting |
|
Light induced water splitting is an attractive way of generating a clean and renewable fuel (hydrogen) from sunlight and water.[1] It is a broad field that will only be successfully implemented in our society with joint efforts of many disciplines, ranging from fundamental inorganic and organic chemistry to material science, nanotechnology, photonics, engineering, among others.
|
|
Evert Jan Meijer
|
Faculty of Science, University of Amsterdam, NL |
|

|
Evert Jan Meijer joined the University of Amstardam in 1998 as a Royal Academy fellow, participating in setting up the Computational Chemistry research theme.
|
Understanding Solvent Effects in Catalysis |
|
|
Rational design of novel efficient catalysts requires a fundamental understanding and accurate quantification of the mechanism, energetics and kinetics of the catalytic cycle. In our approach, we address this challenge by using density functional theory based molecular dynamics (DFT-MD) methods, taking the role of temperature and solvent explicitly into account. |
|
Avner Rothschild
| Department of Materials Science and Engineering, Technion, IL | |
|
Avner Rothschild is a professor at the Department of Materials Science and Engineering of the Technion. He studied physics and materials engineering at the Technion – Israel Institute of Technology, and graduated in 2003 with a PhD on metal-oxide gas sensors. After a three-year postdoc at MIT he returned to the Technion as a faculty member at the Department of Materials Science and Engineering, and the head of the Electrochemical Materials & Devices research group. His research focuses on electrochemical and photoelectrochemical materials and devices for water splitting as a means of sustainable production of green hydrogen from renewable energies such as solar and wind. |
|
Wasted Photons: Photogeneration Yield and Charge Carrier Collection Efficiency of Metal Oxide Photoanodes for Photoelectrochemical Water Splitting |
|
|
At the heart of solar cells is a semiconductor photoabsorber: a material capable of absorbing photons and generating electrons and holes that contribute to the photocurrent. But where commercial photovoltaic cells use silicon for that purpose, photoelectrochemical water splitting cells for solar energy conversion to green hydrogen must rely on other materials that are suitable for operation in aqueous electrolytes. Selected transition metal oxides such as TiO2 and -Fe2O3 (hematite) are known for their stability in alkaline electrolytes, which makes them prime candidates for photoanodes for water photo-oxidation. While transition metal oxides with empty d-shell configurations such as TiO2 (Ti4+ 3d0) and BiVO4 (V5+ 3d0) reach high internal quantum efficiencies, their lower bandgap counterparts with open d-shell configurations such as hematite (Fe3+ 3d5) display low quantum efficiencies that limit their photoconversion efficiency. In the case of hematite photoanodes, less than half of the theoretical photocurrent limit has been reached, despite half a century of research efforts to improve their performance. |
|
Martin Sterrer
|
Institut für Physik, Karl-Franzens Universität Graz, AT |
|
|
Martin Sterrer is the head of the Surface Physics Group at the Institute of Physics at the University of Graz. He obtained his PhD in Physical Chemsitry at Vienna University of Technology under the supervision of Prof. Erich Knözinger. From 2003 to 2006, he worked at the Fritz Haber Institute of the Max Planck Society in Germany as a Postdoctoral fellow with Prof. Hajo Freund. After a stint at the FOM Institute for Atomic and Molecular Physics in Amsterdam in the Biosurface spectroscopy group of Prof. Mischa Bonn, he went back to the Fritz Haber Institute and became the leader of the working group Catalysis/Laser spectroscopy. From 2008 to 2010, Sterrer was a lecturer at the Technische Universität Berlin, where ce received his Habilitation in 2013. He joined the University of Graz as Professor of Experimental Physics in 2014. |
|
Charge Transfer at Metal/Oxide/Organic Interfaces |
|
|
Charge transfer processes through ultrathin, supported oxide films have received increasing attention in recent years because of the possibility to control the charge state of adsorbates or the direction of catalytic reactions. The main driving force for the occurrence of charge transfer in these systems is the reduction of the substrate work function induced by deposition of the oxide film in combination with an adsorbate with high electron affinity. While previous studies have focused on the charging of metal atoms (e.g. Au) or small molecules (e.g. O2, NO2), we have recently extended these investigations to charge transfer processes into organic molecules. In this contribution, we present results on the adsorption and charging of model organic semiconductors (e.g. pentacene (5A), 2H-tetraphenylporphyrin (2H-TPP) and others) on ultrathin MgO(001) films supported on Ag(001). By combing scanning tunneling microscopy and photoemission spectroscopy and tomography, we identify and quantify charge transfer into the organic monolayer film. In addition, we show that by variation of the work function and the MgO thickness it is possible to drive the system into a state where no charge transfer occurs. In the case of 2H-TPP, charge transfer also appears to strongly influence the self-metalation of 2H-TPP to Mg-TPP. Thus, our investigations lay the basis for the ultimate control of charge transfer, and the related chemistry, on ultrathin oxide film systems. |
|
Timmy Ramirez-Cuesta
|
Neutron Sciences, Oak Ridge National Laboratory, USA |
|

|
Timmy Ramirez-Cuesta is the leader of the Chemical Spectroscopy Group at the Oak Ridge National Laboratory. He studied physics in Argentina and completed his PhD Thesis working on computer modeling of chemical reaction on surfaces. He worked at the Rutherford Appleton Laboratory in the UK as a senior instrument scientist at the TOSCA spectrometer. He developed the methodology to calculate the results of dynamical properties calculations. Ramirez-Cuesta is the author of the ICEMAN software and the coauthor of the book Vibrational Spectroscopy with Neutrons: with applications in Chemistry, Materials Science and Catalysis; World Scientific 2005. He joined ORNL in 2013. |
Inelastic Neutron Scattering in Chemistry and Catalysis |
|
|
A wide range of advanced experimental methods has been used in catalytic science to understand molecular behavior in chemical processes. Neutron scattering gives information that is highly complementary from other microscopic scattering techniques such as electrons (microscopy and diffraction) photons from visible light to synchrotron X-rays standard laboratory measurement. |
|
Jenny Zhang
| Department of Chemistry, University of Cambridge, UK | |
Jenny Zhang completed her PhD in bioinorganic chemistry at the University of Sydney, then became a Marie Curie Incoming International Fellow at the University of Cambridge to explore how biocatalysts can be exploited to generate solar fuels. In particular, she worked on developing strategies to re-wire oxidoreductases, such as the water-oxidation enzyme photosystem II, to electrodes/semiconductors/other proteins in an emerging field known as 'semi-artificial photosynthesis'. |
|
Photosynthesis on an Electrode |
|
Photosynthesis is the primary route from which solar energy is harnessed to fuel life on Earth. Underlying this important process are impressive machineries that convert Earth-abundant resources (sunlight, water and carbon dioxide) into complex chemicals. We now have the capability to electrochemically tap into the photosynthetic electron transport chain in vivo and harness its electrons for alternative energy conversion technologies [1, 2]. However, enormous hurdles remain when wiring photosynthetic components to electrodes compared to the wiring of molecular catalysts or enzymes. [3] |
|